
INTENDED USE
*TheAdvancedQualityOne Step Multi-Drug Screen Test is a rapid,qualitative,competitive immunoassay for the determination of Drug- of-Abuse(DOAand/or their metabolites in human urine.
*The device allows the detection of multiple drugs in one simple step.
*The Advanced Quality One Step Multi-Drug Screen Test is intended to be used in professional Medical& Forensic laboratories.
*This test provides only preliminary data which should be confirmed by other methods such as gas chromatography / mass spectrophotometry(GC/MS).
*This test is not intended to monitor drug levels, but only to screen urine for the presence ofthe drugs mentioned and their metabolites.
SUMMARYANDEXPLANATION OF THE TEST
*TheAdvancedQualityOne Step Multi-Drug Screen Test employs unique antibodies to selectivelly identify the following drugs of abuse and/or their metabolites in urine with high degree of sensitivity and specificity:



REAGENTS AND MATERIALS SUPPLIED
(MATERIALS REQUIRED BUT NOT PROVIDED 1 Clock or Timer)
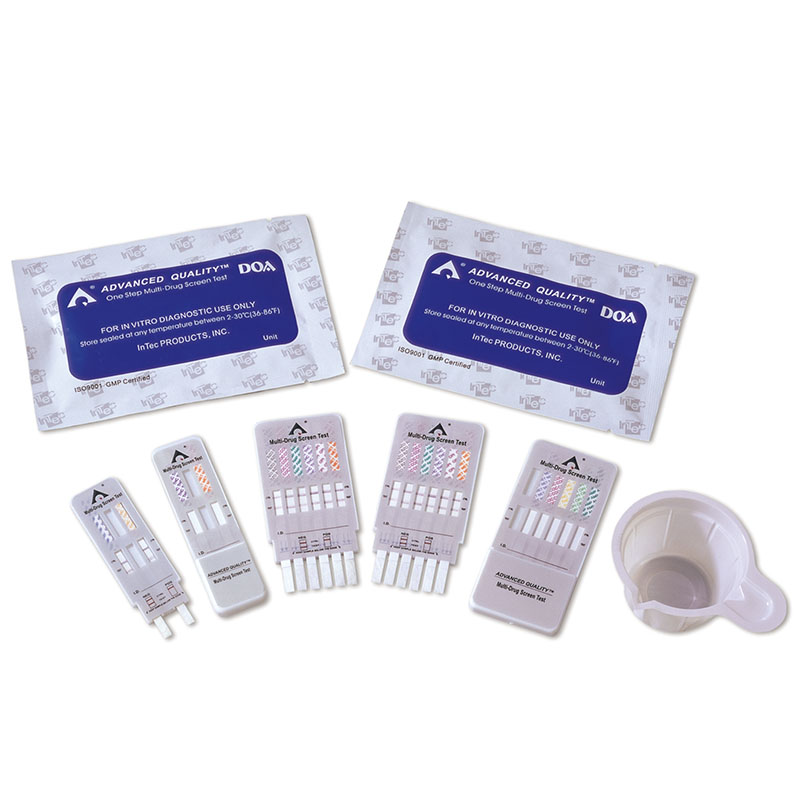
1.Test cards individually foil pouched with a desiccant
2.Urine cups(Optional)
3.Package insert
WARNINGS AND PRECAUTIONS
1.For in vitro diagnostic use only.
2.For professional Medical and Forensic use only
3.Do not use the kit beyond the expiration date imprinted on the outside of the foil pouch.
4.Do not open the foil pouch until the urine is collected and ready to be tested.
5.Avoid cross contamination of urine samples by using a new urine samplle cup for each sample.
6.Urine specimens may be infectious. Upon completion of all testing dispose of residual urine in an approved mannerProperly handle and dispose of all used reaction devices in a biohazard container.
ASSAY PROCEDURE
1.Bring all materials and specimens to room temperature.
2.Remove test device from the sealed foil pouch
3. Remove the cap from the device.
4.Immerse the bottom end of the test strips (the sample pads) into urine sample with the arrows pointing toward the specimen.(keep the level of urine sample below the bottom of plastic card or the maximum line marked on the strips,)
5.Hold the device in the urine until a reddish collor appears at the lower edge of the test membrane (approximatel 10seconds)
6.Withdraw the device from urine sample,and replace the cover7Readresults between 3-8 minutes.
About Us


FAQ
1.Are you factory/manufacturer or trade company?
Yes,We are the direct factory manufacturer who owns productsion lines and workers.and everything is flexible and you don't worry about changing extra money by the middle man or trader.
2.Can you make our design ?
Yes,Your own designs/sketches/pictures are welcoming.
3.Samples available?
Yes,Samples day 3-7days ,more daysif make complicate design .
4.Shipment?
Samples use fast express(UPS,DHL,FEDEX,etc),bulk delivery by air or ship.
5.Payment Terms?



 Silver supplier
Silver supplier








 Facebook
Facebook  Twitter
Twitter  Linkedin
Linkedin  YouTube
YouTube  Blogger
Blogger  Instagram
Instagram 
















